Where to Seek Care When You're Feeling Unwell
Where to Seek Care When You're Feeling Unwell
Use our guide to help determine the appropriate place to seek care for you or your loved one.

Inside the Children's Hospital
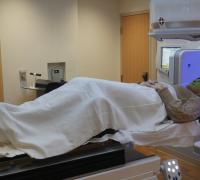
‘Small, But Mighty’
How UVM Children’s Hospital helped save the life of an infant with brain cancer.
News
Events & Programs
daily
7
-8
8
-9
November
21
December
13